Topical mitomycin C application post endoscopic removal of sinonasal inverted papilloma
Matteo Chiarlone 1 , Elena Piumetto 1 , Giovanni Guzzo 1 , Ester Cravero 1 , Lucia Bagnasco 2 , Anna
Morello 2 , Carlo Brunetti 2, Toni Pazzaia 1
1 Unit of Otorhinolaryngology – Head and Neck Surgery, SS.Annunziata Hospital, Savigliano,
Cuneo, Italy
2 Unit of Hospital Pharmacy – SS.Annunziata Hospital, Savigliano, Cuneo, Italy
Correspondence:
Matteo Chiarlone
Unit of Otorhinolaryngology – Head and Neck Surgery, SS.Annunziata Hospital, Via Ospedali 9,
12038 Savigliano, Cuneo, Italy
Tel. +39 0172719368
E-mail: matteo.chiarlone@aslcn1.it
Abstract
Sinonasal inverted papilloma (IP), first described in 1854, is a benign epithelial lesion of the nasal
cavity and paranasal sinuses, composed of well differentiated columnar or ciliated respiratory
epithelium having variable squamous differentiation. The incidence of inverted papilloma has
been reported as 0.2 to 0.7 cases per 100 000 population per year and comprise 0.5 to 4 % of
primary nasal tumours . The most involved sites, in descending order, are: lateral nasal wall,
ethmoid cells, maxillary sinus, and, less often, the frontal and sphenoid sinuses and nasal septum.
Surgery is the first choice in the treatment of inverted papilloma. This study aimed to asses the
effectiveness of mitomycin in reducing inverted papilloma’s volume to undergo a less invasive
surgical approach in order to reduce the risk of intra and post operative complications.1
Keywords
Sinonasal inverted papilloma (IP), endoscopic sinus surgery, mitomycin C, recurrence
Introduction
The World Health Organization (WHO) defines sinonasal inverted papilloma as a benign epithelial
lesion composed of well differentiated columnar or ciliated respiratory epithelium having variable
squamous differentiation. (1)
The WHO classifies IP as a subgroup of Schneiderian papillomas. The term inverted papilloma
describes the histological appearance of the epithelium, i.e. inverting into the stroma, with a
distinct and intact basement membrane that separates and defines the epithelial component from
the underlying connective tissue stroma. (2)
The incidence of IP has been reported as 0.2 to 0.7 cases per 100 000 population per year (3)
Inverted papillomas comprise 0.5 to 4 % of primary nasal tumours. (4) There is no site
predilection, and bilateral cases are seen in 4.9 % of patients. The male to female ratio is 7:1 (5)
with a higher prevalence in the Caucasian race. The peak age of presentation is in the fifth and the
sixth decades (average age, 53 years);
The most common nasal sites involved by inverted papilloma, in order of descending prevalence,
are: lateral nasal wall, ethmoid cells, maxillary sinus, and, less often, the frontal and sphenoid
sinuses and nasal septum. (6) Inverted papilloma originating medially (i.e. from the septum or
turbinates) comprises 34 %of reported cases; that originating laterally (i.e. from the sinuses and
lateral nasal wall) makes up the rest (66 %).(7) The lateral nasal wall represents the most common
site of origin, whereas paranasal sinuses are quite frequently found to be involved by extension.
(8) However, isolated sphenoid involvement with inverted papilloma has been reported in a few
cases . (8-9)
The main characteristics that distinguish it from other sinonasal tumors are: relative local
aggression, high rates of recurrence , whether early or late, and possible association with
carcinoma, diagnosed initially or at recurrence.
Etiology of IP remain mostly unknown. Certain hypotheses have been suggested, but causality has
never been established for the suspected factors: smoking, allergy or certain occupational
exposures (10). Recurrence and carcinomatous potential have for many years suggested viral
origin. An implication of Epstein–Barr Virus (EBV) has been studied, but inconclusively (11). For
more than 30 years, human papilloma virus (HPV) has been suspected of playing a major role in
the pathophysiology of IP, but the literature data remain contradictory (12)
Clinical nonspecific symptoms of IP may include unilateral occlusion, rhinorrhea, sinus infection,
and hyposmia/anosmia. Such primary manifestations may be accompanied by headaches and
facial pain/pressure, lacrimation, or impaired vision (13) Endoscopically, the lesion appears as a
grayish polypoid mass with a multinodular surface.(14) The treatment of choice is surgical; a
purely endoscopic endonasal approach or combined endoscopic and external approach has now
become the gold standard for many authors.(15) Chemotherapy and/or radiation therapy may
follow surgery in case of malignant transformation; many authors have suggested that
radiotherapy could be used for patients whose tumor has not been completely resected or in case
of multiple recurrences.(16)
Mitomycin is derived from Streptomyces caespitosus and has antineoplastic activity similar to that of the
alkylating agents. Mitomycin selectively inhibits the synthesis of DNA by causing cross-linking, degrades
preformed DNA, and causes nuclear lysis and formation of giant cells. At high concentrations, cellular RNA
and protein synthesis may also be suppressed. Mitomycin is cell cycle phase-nonspecific, although it has its
maximum effect in late Gand early S-phases. It is used intravenously to treat uppergastro-intestinal
cancers; however its most common usage is topically. Both human and animal applications
confirm the safety of topical use of mitomycin-C (17,18)
It has many topical usages by otolaryngologist in endoscopic sinus surgery, (19) choanal
atresia,(20) laryngeal,(21) and tracheal surgery(22) to minimize or even prevent post-operative
synechia and stenosis.
The aim of this study was to assess the effectiveness of mitomycin in reducing the size of IP
recurrence after endoscopic endonasal approach.
Materials and methods
In January 2020 a 52 years old female, without comorbidities, underwent to ent examination for
sinonasal symptoms: unilateral nasal obstruction, anterior and/or posterior rhinorrhea
and hyposmia . Clinical examination by endoscopic exploration found, in the left nasal cavity, a
reddish-gray lobulated tumor, more firm than an inflammatory polyp.
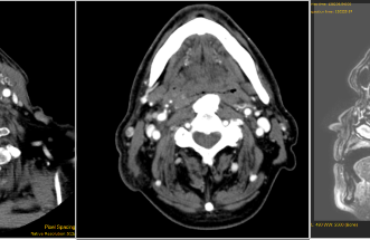
The patient underwent a CT scan and a MRI as a complement to CT.
Subsequently, endoscopic biopsy was performed with the histogical diagnosis of pT3 inverted
papilloma (according to Krouse's classification).
According to literature data, a pT3 inverted papilloma (according to Krouse's classification)
extended up to the anterior wall of the maxillary sinus would require extensively demolitive
surgical treatment capable of widely exposing the maxillary sinus up to its anterior wall.
In these cases, a type III median maxillectomy ( also called "Sturmann-Canfield" surgery in which
the maxillectomy is enlarged anteriorly to include the nasolacrimal duct up to the exposure of the
piriform notch) is generally proposed.
This type of surgical intervention was proposed to the patient and the risks and complications
related to surgery was explained: surgical approach was refused.
After an extensive informative interview with the patient and after authorization of the local
ethics committee, it was decided to proceed with local dressings with 5% Mitomycin once a month
for 6 months.
Therefore, starting from October 2020, the patient was subjected to local dressings under
endoscopic guidance in local anesthesia: during the dressings, after local anesthesia, the edged
gauze was placed at the level of the left maxillary cavity. Subsequently it was soaked with 5%
Mitomycin. The dressing was left in place for 30 minutes and then removed.
This dressing was repeated in November 2020, December 2020 and January 2021.
At the endoscopic follow-up in January 2021 there was a clear reduction in the hyperplastic area
reported in October 2020. Therefore, in agreement with the patient, it was decided to practice
two other dressings (February 2021 and March 2021) and to evaluate, subsequently, endoscopic
revision surgery which, in consideration of the volumetric reduction of the lesion, will certainly be
less invasive than expected at the beginning.
In our case, following the topical chemotherapy treatment with mitomycin, we obtained a
complete reabsorption of the PI at the level of the anterior wall and the zygomatic process.
Therefore we decided to plan a type II median maxillectomy with preservation of the nasolacromal
duct. In order to dominate the anterior wall of the left maxillary sinus more easily, we enlarged the
maxillectomy anteriorly keeping ourselves under the Hasner valve, preserving the nasolacrimal
duct: this allowed us to perform an easy maxillary sinusoscopy also at the level of the anterior wall
thanks to the use of a 70° optic.
Contrary to what appeared from the radiological investigation performed before the treatment
with mitomycin, we found an absence of involvement of the anterior maxillary wall by the IP.
Therefore, having observed a PI limited to the posterior and medial walls, we decided not to
perform a type III maxillectomy. Furthermore, since the neoformation extended to the anterior
third of the middle meatus, we performed a subtotal resection of the first portion of the middle
turbinate, a total uncinectomy with opening of the frontal pre-chambers and the opening of the
bulla ethmoidalis. We performed opening of the second portion of the middle turbinate and
subsequent posterior ethmoidectomy (surgical time 120 minutes)
We also greatly reduced the risk of post-operative complications compared to a type III
maxillectomy
The postoperative course was regular and uneventful. The patient was subjected to periodic
(monthly) checks for about 6 months. During the checks, nasal endoscopy under local anesthesia
was performed. No signs of disease recurrence were found during the controls.
Discussion
The main treatment of IP is surgery, and the cornerstone of successful surgery is the radical
exeresis of the lesion.
However there is a high risk of recurrence especially for lesions located in areas that are difficult to
control with the surgical endoscopic approach.
In the current study we used the MMC application to decrease the the volume of the lesion and
minimal morbidly effect of transnasal endoscopic approach.
Mitomycin-C (MMC) has also many topical usages in otolaryngology. It was used safely to reduce
nasal synechiae and stenosis after endoscopic sinus surgery,(10) and choanal atresia (11).
The etiopathogenesis of the IP is still not well understood but this effect of MMC in minimizing IP
volume could be attributed to that the MMC is a chemotherapeutic agent that inhibits the DNA
synthesis and so inhibits cell migration and mitosis decreasing the rate of cell proliferation.
MMC was applied easily and safely without any added risk to the patients; no granulations, crusts,
infection, bleeding was reported in the clinical case with its usage.
The technique proved to be safe, reliable, easily applicable and effective.
In particular, we demonstrated how the use of mitomycin was useful in order to reduce the
volume of the lesion and make the following endoscopic surgical treatment less invasive.
Conclusion
Topical MMC application in nasosinusal inverted papilloma, is safe and effective in reducing the volume of
the lesion and planning less invasive endoscopic surgical treatments in order to reduce intra and post-
operative complications.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding acengies in the public, commercial of
not-for-profit sectors.
Author contributions
MC, EP performed manuscript preparation; EP, EC performed final edits and revisions; LB, AM, CB
read and approved the submitted version.
References
1. Eggers G, Mühling J, Hassfeld S. Inverted papilloma of paranasal sinuses. J Craniomaxillofac Surg
2007 Jan;35(1):21-9. https://doi.org/10.1016/j.jcms.2006.10.003
2. Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: recurrence, and
synchronous and metachronous malignancy. J Laryngol Otol. 2007 Sep;121(9):857-64. https://doi:
10.1017/S002221510700624X
3. Buchwald C, Franzmann MB, Tos M. Sinonasal papillomas: a report of 82 cases in Copenhagen
County, including a longitudinal epidemiological and clinical study. Laryngoscope. 1995
Jan;105(1):72-9. https://doi: 10.1288/00005537-199501000-00016
4. Lane AP, Bolger WE. Endoscopic management of inverted papilloma. Curr Opin Otolaryngol Head
Neck Surg. 2006 Feb;14(1):14-8. https://doi: 10.1097/01.moo.0000193175.54450.1f
5. Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma.
Laryngoscope. 1995 Feb;105(2):178-83. https://doi: 10.1288/00005537-199502000-00013
6. Krouse JH. Endoscopic treatment of inverted papilloma: safety and efficacy. Am J Otolaryngol. 2001
Mar-Apr;22(2):87-99. https://doi: 10.1053/ajot.2001.22563
7. Lee TJ, Huang CC, Chen YW, et al. Medially originated inverted papilloma. Otolaryngol Head Neck
Surg. 2009 Mar;140(3):324-9. https://doi: 10.1016/j.otohns.2008.10.037.
8. Yiotakis I, Psarommatis I, Manolopoulos L, et al. Isolated inverted papilloma of the sphenoid sinus. J
Laryngol Otol. 2001 Mar;115(3):227-30. https://doi: 10.1258/0022215011907046.
9. Fakhri S, Citardi MJ, Wolfe S, et al. Challenges in the management of sphenoid inverted papilloma.
Am J Rhinol. 2005 Mar-Apr;19(2):207-13. Erratum in: Am J Rhinol. 2005 Jul-Aug;19(4):422.
10. Barnes L, Eveson J, Reichart P, et al. World Health Organization Classification of Tumours.
Pathology and genetics of head and neck tumours IARC Press, Lyon (2005)
11. Buchwald C, Franzmann MB, Tos M. Sinonasal papillomas: a report of 82 cases in Copenhagen
County, including a longitudinal epidemiological and clinical study. Laryngoscope. 1995
Jan;105(1):72-9. https://doi: 10.1288/00005537-199501000-00016.
12. Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal papillomas: systematic
review and meta-analysis. Laryngoscope. 2013 Jan;123(1):181-92. https://doi: 10.1002/lary.23688.
13. Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma.
Laryngoscope. 1995 Feb;105(2):178-83. https://doi: 10.1288/00005537-199502000-00013.
14. Wang MJ, Noel JE. Etiology of sinonasal inverted papilloma: A narrative review. World J
Otorhinolaryngol Head Neck Surg. 2016 Dec 21;3(1):54-58. https://doi:
10.1016/j.wjorl.2016.11.004.
15. Peng R, Thamboo A, Choby G, et al. Outcomes of sinonasal inverted papilloma resection by surgical
approach: an updated systematic review and meta-analysis. Int Forum Allergy Rhinol. 2019
Jun;9(6):573-581. https://doi: 10.1002/alr.22305.
16. Miyazaki T, Haku Y, Yoshizawa A, et al. Clinical features of nasal and sinonasal inverted papilloma
associated with malignancy. Auris Nasus Larynx. 2018 Oct;45(5):1014-1019. https://doi:
10.1016/j.anl.2018.02.009.
17. Warner D, Brietzke SE. Mitomycin C and airway surgery: how well does it work? Otolaryngol Head
Neck Surg. 2008 Jun;138(6):700-9. https://doi: 10.1016/j.otohns.2008.02.011.
18. Crosara PF, Vasconcelos AC, Guimarães RE, Becker HM, Becker CG, Crosara SL, Nascimento E. Effect
of mitomycin C on the secretion of granulocyte macrophages colonies stimulating factor and
interleukin-5 in eosinophilic nasal polyps stromal culture. Braz J Otorhinolaryngol. 2005 Jul-
Aug;71(4):459-63. https://doi: 10.1016/s1808-8694(15)31199-x..
19. Konstantinidis I, Tsakiropoulou E, Vital I, et al. Intra- and postoperative application of Mitomycin C
in the middle meatus reduces adhesions and antrostomy stenosis after FESS. Rhinology. 2008
Jun;46(2):107-11.
20. Carter JM, Lawlor C, Guarisco JL. The efficacy of mitomycin and stenting in choanal atresia repair: a
20 year experience. Int J Pediatr Otorhinolaryngol. 2014 Feb;78(2):307-11. https://doi:
10.1016/j.ijporl.2013.11.031.
21. Hueman EM, Simpson CB. Airway complications from topical mitomycin C. Otolaryngol Head Neck
Surg. 2005 Dec;133(6):831-5. https://doi: 10.1016/j.otohns.2005.07.031.
22. Reichert LK, Zhao AS, Galati LT, et al. The Efficacy of Mitomycin C in the Treatment of
Laryngotracheal Stenosis: Results and Experiences with a Difficult Disease Entity. ORL J
Otorhinolaryngol Relat Spec. 2015;77(6):351-8. https://doi: 10.1159/000439174. Epub 2015 Oct 10.