The clinical challenge of dealing with laryngopharyngeal reflux symptoms: a prospective observational study on a new nutraceutical formulation (CHETOGERD®)
Giulia Fiorini1, Matteo Pavoni2, Matteo Fermi3, Giulia Molinari3, Andrea Imbrogno4, Attilio Varicchio5, Ignazio La Mantia6, Claudio Borghi1,2, Gabriele Molteni2,3, Dino Vaira1,2,* and Livio Presutti2,3
- Cardiovascular Medicine Unit, Heart, Chest and Vascular Dept., IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- Department of Medical and Surgical Sciences, University of Bologna, 40138 Bologna, Italy.
- Department of Otolaryngology – Head and Neck Surgery, IRCCS Azienda Ospedaliero-Universitaria di Bologna
- U.O.C Pronto Soccorso ed Emergenza Territoriale AUSL Bologna Area Spoke, Bologna, Italy
- Department of Otolaryngology-Head and Neck Surgery, “V.Tiberio” Univerisity of Molis, Italy
6. Department Headf Otolaryngology – Head and Neck Surgery, University of Catania, Italy
*Corresponding author: Prof. Dino Vaira, Department of Surgical and Medical Sciences, University of Bologna, Bologna, Italy, Phone/fax: +39 051-2144140 e-mail: berardino.vaira@unibo.it
Abstract
Background and aim: in the last years, many investigators have proposed an association between gastroesophageal reflux disease and laryngopharyngeal symptoms such as hoarseness, globus pharyngeus, vocal fatigue, frequent sore throat, frequent throat clearing, chronic cough. As reflux is implicated in the pathogenesis of symptoms, it has been suggested that medical or surgical therapy may be helpful to manage these patients. The study aimed to evaluate the efficacy of a new nutraceutical formulation (CHETOGERD®) in inducing remission of laryngopharyngeal symptoms. Methods: The ENT specialist selected patients with laryngopharyngeal symptoms of suspected gastroesophageal etiology and consecutively sent them to the gastroenterologist. Patients found to be Helicobacter pylori-negative were assessed at baseline and after two months of treatment to evaluate the frequency and severity of extraesophageal reflux symptoms with the reflux finding score (RFS). Conclusion: CHETOGERD® formulations were able to achieve a statistically significant reduction of severity and frequency of symptoms analyzed by the RFS at 0 and 2 months. Our study underlines the need for a different therapeutic approach in patients with laryngoesophgeal reflux symptoms to reduce the number of visits and unnecessary therapies.
Keywords: laryngopharyngeal reflux symptoms, Helicobacter pylori, nutraceutical formulations.
Introduction
In recent years, many investigators have proposed an association between gastroesophageal reflux disease (GERD) and laryngopharyngeal symptoms such as hoarseness, globus pharyngeus, vocal fatigue, sore throat, frequent throat clearing, and chronic cough [1].
Several mechanisms have been investigated, such as acid stimulation of vagal afferents in the distal and/or proximal esophagus and direct laryngeal contact with acid, pepsin, or other substances present in gastroesophageal reflux [2]. It is estimated that acid reflux-related laryngitis has ranged from 18 to 80% in the last two decades. Moreover, extraesophageal symptoms are often the only ones capable of highlighting the presence of reflux [3].
As reflux is implicated in the pathogenesis of symptoms, it has been suggested that medical or surgical therapy may be helpful to manage these patients [4].
The standard management of GERD is usually based on the frequency and severity of symptoms, ranging from lifestyle and dietary modification to the use of proton pump inhibitors (PPIs) if symptoms are still not under control. [5] PPIs are the mainstay of treatment for oesophageal manifestations, but alternative strategies have been considered with evidence of long-term side effects of cumulative PPI use over time [6,7].
Recently, the use of nutritional compounds has been proposed as an alternative for patients with gastroesophageal reflux disease, especially those with symptoms such as heartburn and reflux without visible esophageal mucosal damage defining the so-called “non-erosive reflux disease” (NERD) [8].
These studies have shown that the use of CHETOGERD® gel or oro formulations, an association of natural active ingredients (hyaluronic acid, Altea, Malva, apple active TM, aloe vera, L-tryptophan, calcium gluconate, sodium bicarbonate, Musa paradisiaca) may be an alternative or coadjuvant treatment in patients with non-erosive reflux disease [5].
It is, therefore, reasonable to think that these formulations could be helpful in controlling laryngopharyngeal reflux symptoms and atypical manifestations of gastroesophageal reflux disease, reducing the number of ENT visits and improving patients’ quality of life.
Therefore, we planned this unique prospective interventional study to evaluate the efficacy and safety of CHETOGERD® formulations for treating laryngopharyngeal symptoms of suspected gastroesophageal etiology.
Methods
The study aimed to evaluate the efficacy of CHETOGERD® (buccal tablets or gel formulation) in inducing remission of symptoms in patients with laryngopharyngeal symptoms of suspected gastroesophageal etiology.
Exclusion criteria were: the presence of Helicobacter pylori infection; use of proton pump inhibitors or H2 receptor antagonists in the previous four weeks; comorbid conditions (i.e., chronic renal failure, liver cirrhosis); active malignant neoplasm; pregnancy or lactation and inability to sign an informed consent.
Patients presented with laryngopharyngeal symptoms such as hoarseness, globus pharyngeus, vocal fatigue, sore throat, frequent throat clearing, and chronic cough of suspected gastroesophageal etiology for at least four weeks were selected by the ENT specialist and consecutively sent to the gastroenterologist. They all underwent a 13C-urea breath test to rule out Helicobacter pylori infection.
Patients found to be Helicobacter pylori-negative were enrolled and treated with three doses of nutraceuticals/day after meals for two months, as shown in Figure 1.
Figure 1: study flow-chart
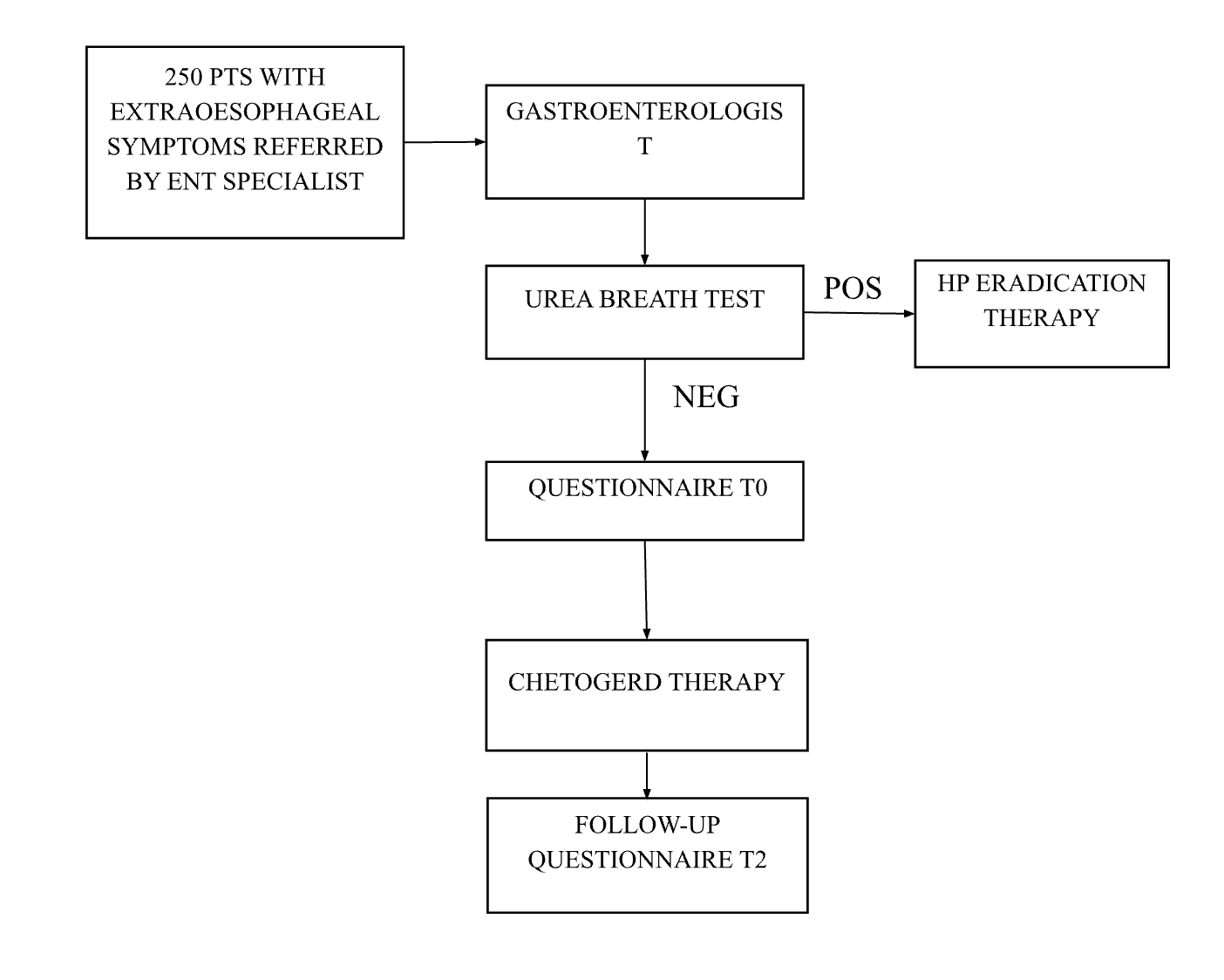
Each dose consisted of a combination of nutraceutical compounds (apple juice, sodium alginate, L-tryptophan, sodium hyaluronate, aloe vera, Musa paradisiaca, calcium gluconate plus preservatives, flavorings, sweetener, and acidity regulators) in liquid or tablets formulation.
Negative patients were assessed at baseline and after two months of treatment to evaluate the frequency and severity of extraesophageal reflux symptoms referred to in the past month with the reflux finding score (RFS) [9]. The severity score ranges from no symptoms (score 0) to severe (score 5), while frequency ranges from 0 (never) to 7 (daily).
Statistical analysis
The study was designed to have a 90% confidence level with a margin of error of 5%. Frequency, distribution analyses, and non-parametric tests were used for the statistical analysis (Medcalc19.1.3 software) on patients who completed both follow-ups at two months. Results were considered statistically significant for p values <0.05.
Results
Two hundred and fifty patients with laryngopharyngeal symptoms of suspected gastroesophageal etiology were consecutively sent for gastroenterological evaluation by the ENT specialist at Sant’Orsola Hospital, Bologna, Italy. Two hundred and sixteen patients (80 males; mean age: 52.7 years) were found to be Helicobacter pylori-negative and were then treated with CHETOGERD® formulations, while thirty-four resulted Helicobacter pylori-positive and were treated for the gastric infection. Patient characteristics are summarized in Table 1. At two months, all 216 patients were followed up.
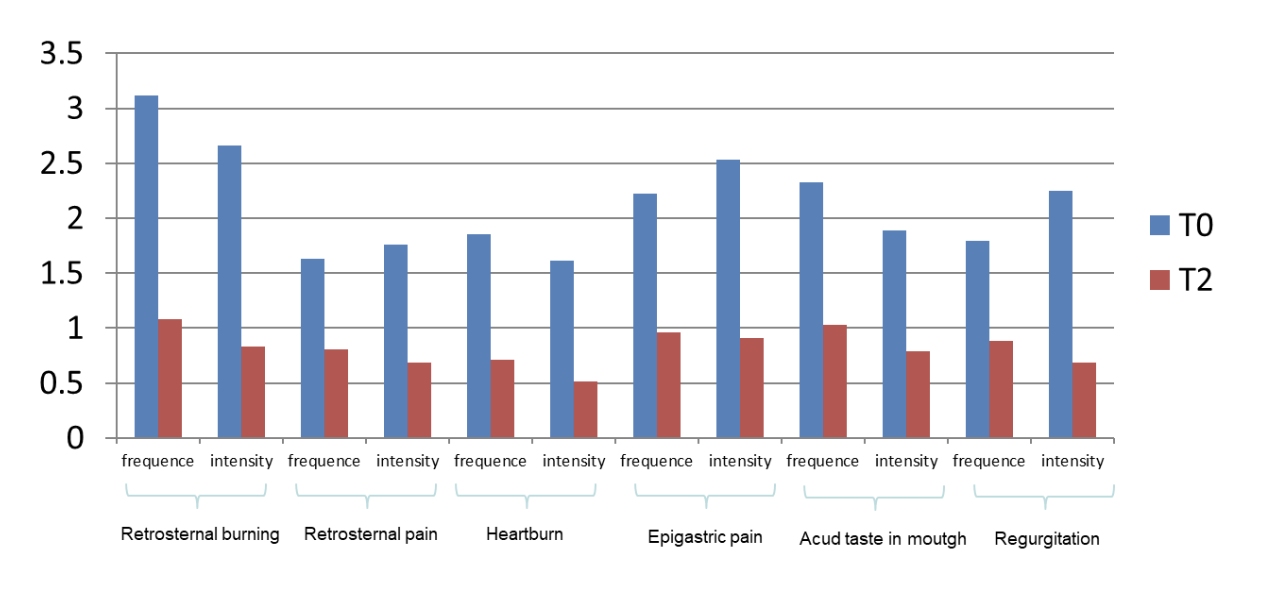
CHETOGERD® formulations were able to achieve a statistically significant reduction of severity and frequency of symptoms analyzed by the reflux finding score (RFS) at 0 and 2 months (p values <0.05) (Figure 2). No difference was observed between normal weight (BMI ? 25) and mild to severe overweight (BMI > 25) patients.
Figure 2: frequency and intensity of symptoms before and after treatment with CHETOGERD®
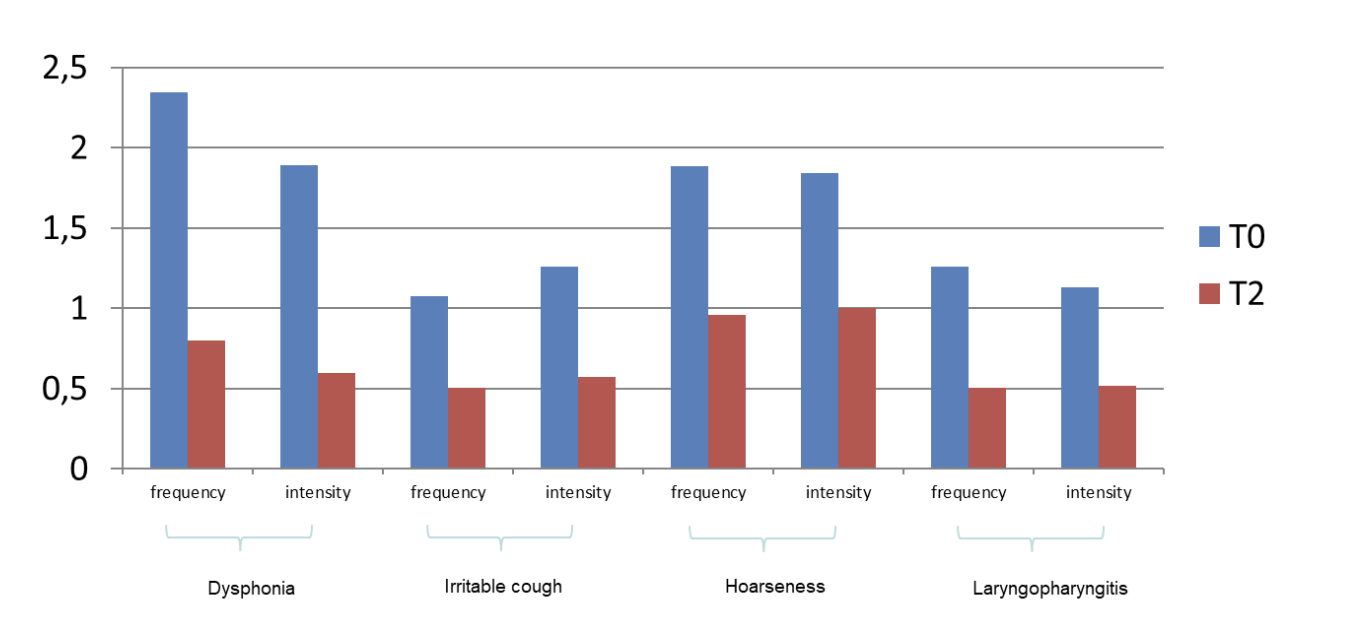
On admission, among extra gastroesophageal symptoms (dysphonia, irritable cough, hoarseness, laryngopharyngitis), dysphonia was the most declared. After two months of CHETOGERD® treatment, a statistically significant reduction in every extra gastroesophageal symptom was observed; in particular, dysphonia showed the most significant decrease (Figure 3).
Figure 3: frequency and intensity of Extra-gastroesophageal symptoms before and after treatment with CHETOGERD®
Discussion
Laryngopharyngeal reflux has been classically referred to as gastroesophageal reflux, leading to chronic laryngeal symptoms such as throat clearing, dysphonia, cough, globus sensation, laryngopharyngitis, hoarseness or mucus in the throat [10].
Since reflux disease is one of the most prevalent health-related conditions in the Western world and it affects the quality of life with high healthcare costs and lost productivity, several attempts to find the right therapeutic approach have been made to treat reflux and associated symptoms [11].
However, patients with chronic laryngeal symptoms are complex due to the heterogeneous nature of symptom pathology, inconsistent definitions, and variable responses to therapies. Moreover, they usually undergo several medical evaluations before obtaining a definite diagnosis and treatment [12].
Despite solid evidence regarding the efficacy of PPIs in the treatment of severe GERD and its complications, there is still conflicting information regarding the dosage and duration of therapy to control extraesophageal symptoms, and the current lack of clear diagnostic criteria significantly impairs practitioners’ ability to identify and manage laryngopharyngeal reflux [13].
Recently, new therapeutic approaches have been proposed, which, by acting with different and combined mechanisms, can control or improve symptoms [14]. In this scenario, our study shows that CHETOGERD® formulations were able to achieve a statistically significant reduction of severity and frequency of symptoms in patients with laryngopharyngeal symptoms of gastroesophageal etiology without any documented side effects.
The efficacy of this nutraceutical compound is not only related to the statistically significant benefit on symptoms but also to the rapidity of obtaining them, as patients showed relief of symptoms after only two months of therapy.
These effects could be explained by the combination of its components: hyaluronic acid, which favors the healing process and creates a barrier over the esophageal and gastric mucosae [15]; Altea and malva, which exert a soothing action and help to improve digestive function [16]; apple active TM and aloe vera, that are able to reduce inflammation process [17]; L-tryptophan, that improves gastrointestinal peristalsis [18]; calcium gluconate and sodium bicarbonate, that act in contrast to gastric acidity in combination with musa paradisiaca that helps to increase oesophageal barrier by increasing mucus production [19].
Despite the promising results achieved, this study has several limitations: firstly, the observational and non-blinded design does not allow the results to be safely extended to a larger population. Secondly, the reported symptoms may have attenuated due to the placebo effect connected to the treatment process. Lastly, the absence of a direct comparison with the therapeutic gold standard does not allow us to define the treatment as a stable therapeutic alternative. In any case, this study demonstrates the possibility of improving patients’ symptoms through a correct definition of the etiology of the symptoms and, therefore, through a treatment more targeted at the gastrointestinal problem than at the ENT one.
Moreover, our study underlines the need for a different therapeutic approach in patients with laryngoesophgeal reflux symptoms to reduce the number of visits and unnecessary therapies.
Although these data are promising, further research is needed to confirm the efficacy and safety of CHETOGERD® and to better understand its potential in the treatment of extraesophageal reflux disease.
Author Contributions: Conceptualization: Professor Dino Vaira. Dino Vaira and Professor Livio Presutti. Giulia Molinari, Matteo Fermi and Gabriele Molteni included patients in the study. Matteo Pavoni performed data management and statistical analysis. Giulia Fiorini and Matteo Pavoni wrote the manuscript. All authors (Giulia Fiorini, Matteo Pavoni, Matteo Fermi, Giulia Molinari, Andrea Imbrogno, Attilio Varicchio, Ignazio La Mantia, Claudio Borghi, Gabriele Molteni, Dino Vaira and Livio Presutti) critically reviewed the manuscript and approved the final version of this manuscript. All authors read and approved the final version of the manuscript.
Funding: This research received no external funding.
Statement of human and animal rights: The study was carried out according to Good Clinical practice guidelines.
Conflicts of Interest: The authors declare no conflict of interest.
Table 1: population characteristics
| Population (216 patients) | N° | % | 95%IC |
| Male | 80 | 37.1 | 30.9-43.7 |
| Female | 136 | 62.9 | 56.4-69.1 |
| Age range (mean) | 18-92, (52.7) | // | // |
| BMI range (mean) | 15.6-43.0 (24.8) | // | // |
| Active smokers | 33 | 15.3 | 11.1-20.7 |
| Alcohol consumption (at least 1 glass a day) | 29 | 13.4 | 9.5-18.6 |
| NSAIDS (in the last 2 weeks) | 17 | 7.9 | 5.0-12.2 |
REFERENCE
- Gatta L, Vaira D, Sorrenti G et al. Meta-analysis: the efficacy of proton pump inhibitors for laryngeal symptoms attributed to gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007 Feb 15;25(4):385-92. doi: 10.1111/j.1365-2036.2006.03213.x. PMID: 17269993.
- Orlando RC. Pathogenesis of gastroesophageal reflux disease. Gastroenterol Clin North Am. 2002 Dec;31(4 Suppl):S35-44. doi: 10.1016/s0889-8553(02)00037-7. PMID: 12489469.
- Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and management laryngopharyngeal reflux disease: state of the art review. Otolaryngol Head Neck Surg. 2019 May;160(5):762-782. doi: 10.1177/0194599819827488. Epub 2019 Feb 12. PMID: 30744489.
- Dutta U, Moayyedi P. Management of reflux-related symptoms. Best Pract Res Clin Gastroenterol. 2013 Jun;27(3):387-400. doi: 10.1016/j.bpg.2013.06.004. PMID: 23998977.
- Fiorini G, Saracino IM, Pavoni M, et al. Efficacy of a new nutraceutical formulation (CHETOGERD) in patients with non erosive reflux disease (NERD): a prospective observational study. Intern Emerg Med. 2020 Oct;15(7):1265-1269. doi: 10.1007/s11739-020-02309-z. Epub 2020 Mar 20. PMID: 32198722.
- Seo SI, Park CH, You SC, et al. Association between proton pump inhibitor use and gastric cancer: a population-based cohort study using two different types of nationwide databases in Korea. Gut. 2021Nov;70(11):2066-2075. doi: 10.1136/gutjnl-2020-323845. Epub 2021 May 11. PMID: 33975868.
- Abrahami D, McDonald EG, Schnitzer ME, et al. Proton pump inhibitors and risk of colorectal cancer. Gut. 2022 Jan;71(1):111-118. doi: 10.1136/gutjnl-2021-325096. Epub 2021 Jul 1. PMID: 34210775.
- Panahi Y, Khedmat H, Valizadegan G, et al. Efficacy and safety of Aloe vera syrup for the treatment of gastroesophageal reflux disease: a pilot randomized positive-controlled trial. J Tradit Chin Med. 2015 Dec;35(6):632-6. doi: 10.1016/s0254-6272(15)30151-5. PMID: 26742306.
- Lukaschyk J, Abel J, Brockmann-Bauser M, et al. The Relation Between Endoscopic and Subjective Laryngopharyngeal Reflux Signs, Vocal Tract Discomfort, Voice Handicap, and Voice Disorder Type: Same Yet Different? J Voice. 2024 Jan 4:S0892-1997(23)00381-8. doi: 10.1016/j.jvoice.2023.11.021. Epub ahead of print. PMID: 38182496.
- Barrett CM, Patel D, Vaezi MF, et al. Laryngopharyngeal Reflux and Atypical Gastroesophageal Reflux Disease. Gastrointest Endosc Clin N Am. 2020 Apr;30(2):361-376. doi: 10.1016/j.giec.2019.12.004. Epub 2020 Jan 22. PMID: 32146951.
- Hessler LK, Xu Y, Shada AL, et al. Antireflux surgery leads to durable improvement in laryngopharyngeal reflux symptoms. Surg Endosc. 2022 Jan;36(1):778-786. doi: 10.1007/s00464-020-08279-9. Epub 2021 Feb 2. PMID: 33528667.
- Silva ÁS, Duprat AC, Machado SR, et al. Evaluation of the Reflux Symptom Index and the Endolaryngeal Findings Scale after Treatment in Individuals with Laryngopharyngeal Reflux. Int Arch Otorhinolaryngol. 2021 Jan;25(1):e115-e122. doi: 10.1055/s-0040-1702967. Epub 2020 May 4. PMID: 33542761; PMCID: PMC7851361.
- Gyawali PC, Kahrilas PJ, Savarino E et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018 Jul;67(7):1351-1362. doi: 10.1136/gutjnl-2017-314722. Epub 2018 Feb 3. PMID: 29437910; PMCID: PMC6031267.
- Lechien JR. Personalized Treatments Based on Laryngopharyngeal Reflux Patient Profiles: A Narrative Review. J Pers Med. 2023 Oct 31;13(11):1567. doi: 10.3390/jpm13111567. PMID: 38003882; PMCID: PMC10671871.
- Savarino V, Pace F, Scarpignato C, the Esoxx Study Group et al. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non?erosive reflux disease – efficacy of Esoxx, a hyaluronic acid–chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017 Mar;45(5):631-642. doi: 10.1111/apt.13914. Epub 2017 Jan 24. PMID: 28116754; PMCID: PMC5347926.
- Salehi M, Karegar-Borzi H, Karimi M, et al. Medicinal Plants for Management of Gastroesophageal Reflux Disease: A Review of Animal and Human Studies. J Altern Complement Med. 2017 Feb;23(2):82-95. doi: 10.1089/acm.2016.0233. Epub 2016 Dec 20. PMID: 27996295.
- Panahi Y, Khedmat H, Valizadegan G, et al. Efficacy and safety of Aloe vera syrup for the treatment of gastroesophageal reflux disease: a pilot randomized positive-controlled trial. J Tradit Chin Med. 2015 Dec;35(6):632-6. doi: 10.1016/s0254-6272(15)30151-5. PMID: 26742306.
- Pereira R de S. Regression of gastroesophageal reflux disease symptoms using dietary supplementation with melatonin, vitamins and aminoacids: comparison with omeprazole. J Pineal Res. 2006 Oct;41(3):195-200. doi: 10.1111/j.1600-079X.2006.00359.x. PMID: 16948779.
- Alese MO, Adewole SO, Akinwunmi KF, et al. Aspirin-Induced Gastric Lesions Alters EGFR and PECAM-1 Immunoreactivity in Wistar Rats: Modulatory Action of Flavonoid Fraction of Musa Paradisiaca. Open Access Maced J Med Sci. 2017 Jul 26;5(5):569-577. doi: 10.3889/oamjms.2017.058. PMID: 28932294; PMCID: PMC5591583.